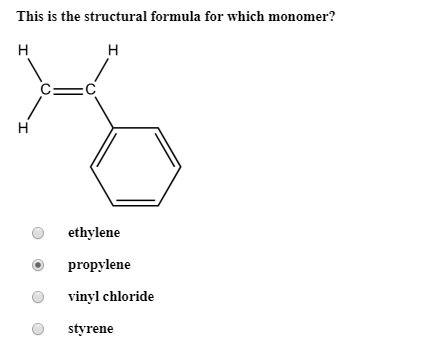
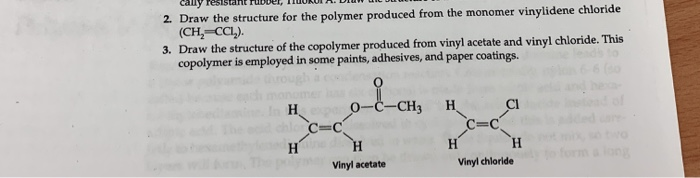
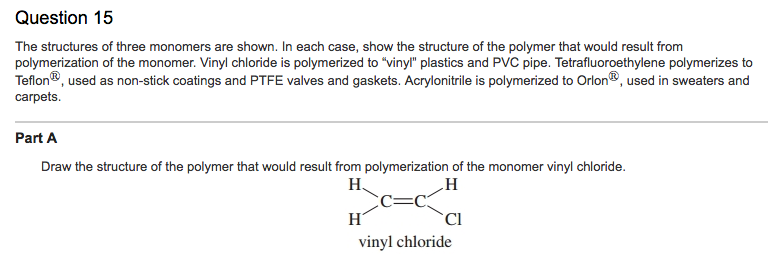
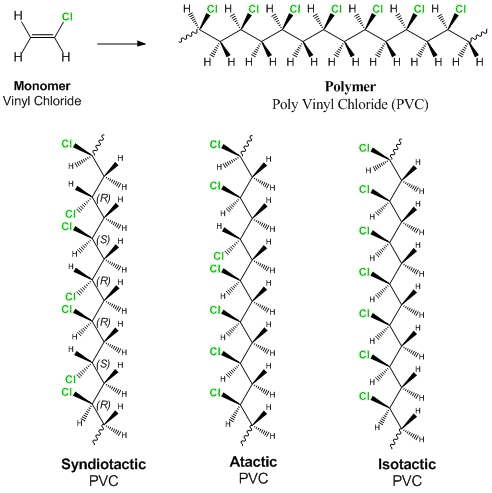
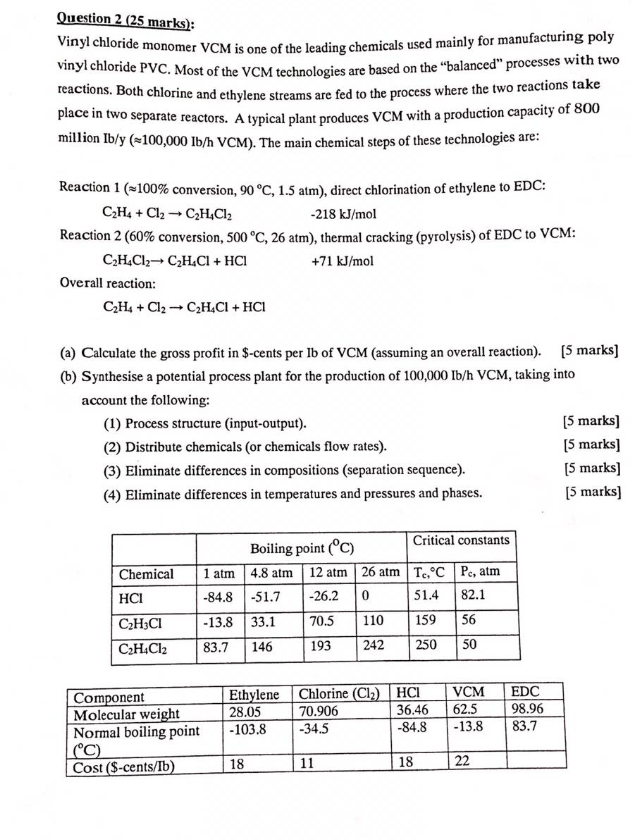
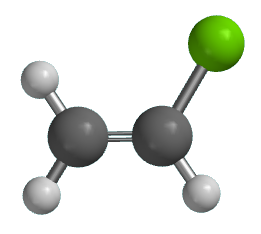
Vinyl Chloride Monomer Structure
Vinyl chloride is a chlorinated hydrocarbon occurring as a colorless highly flammable gas with a mild sweet odor that may emit toxic fumes of carbon dioxide carbon monoxide hydrogen chloride and phosgene when heated to decomposition.
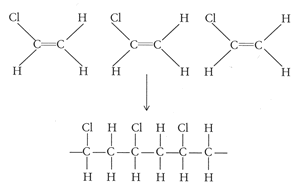
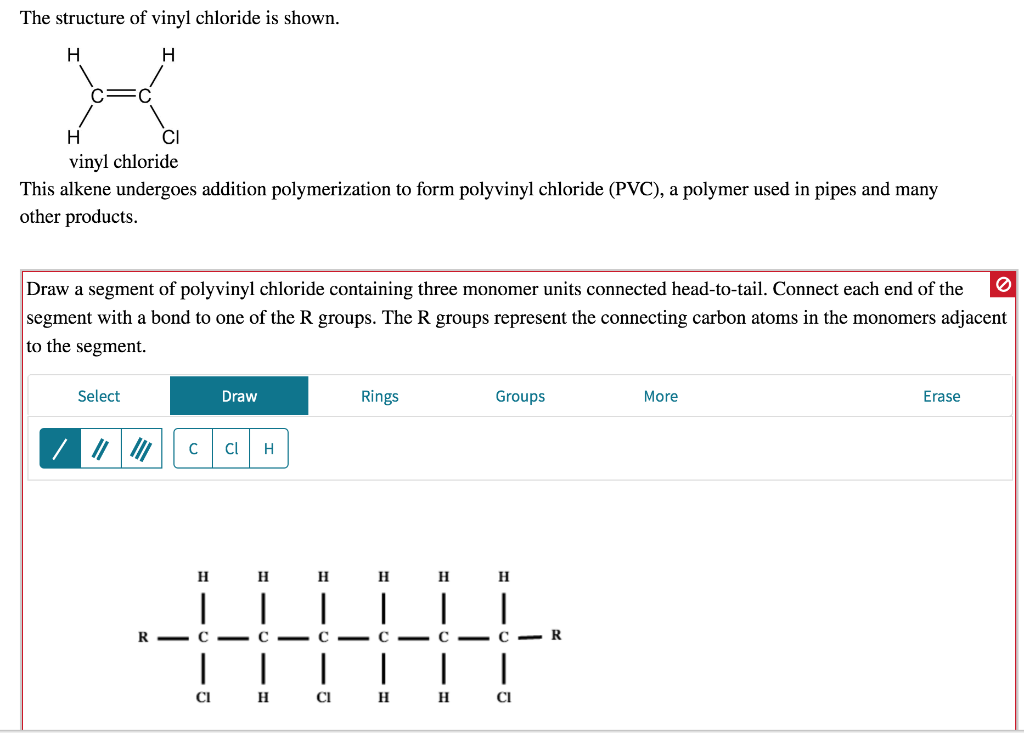
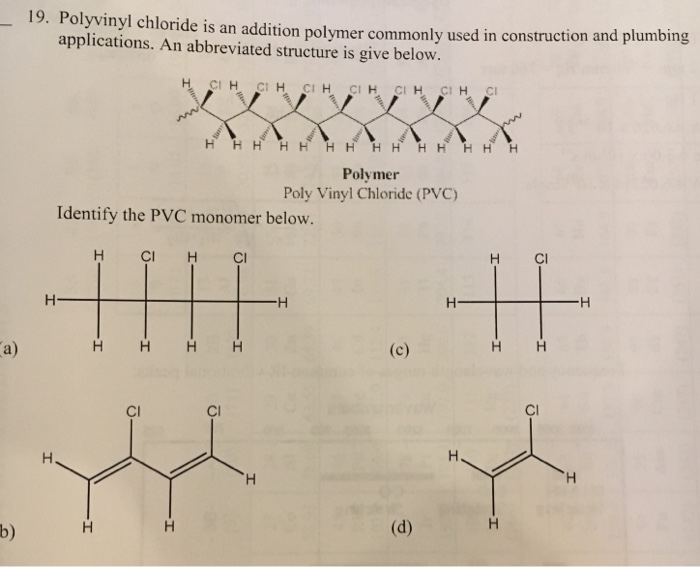
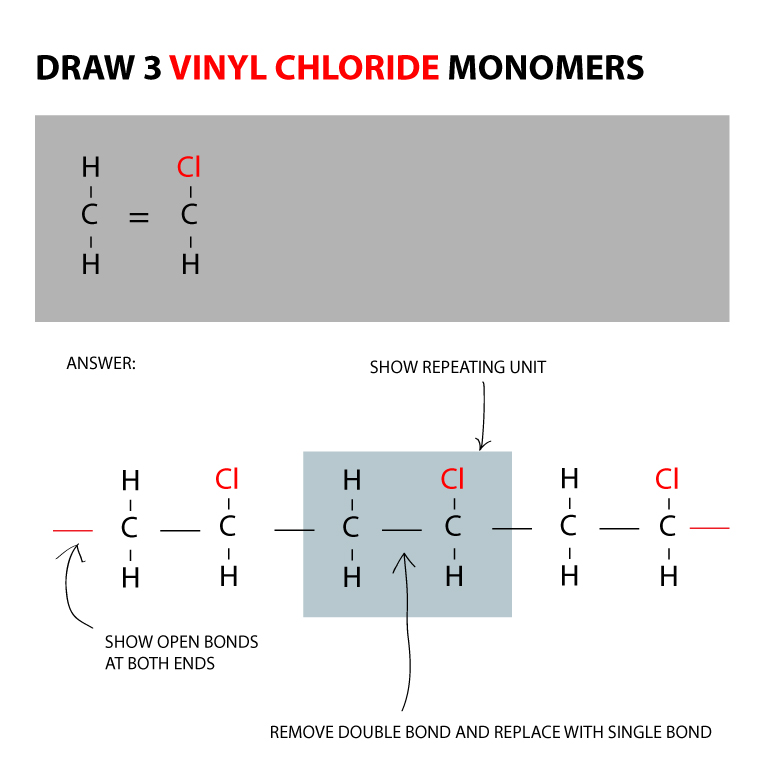
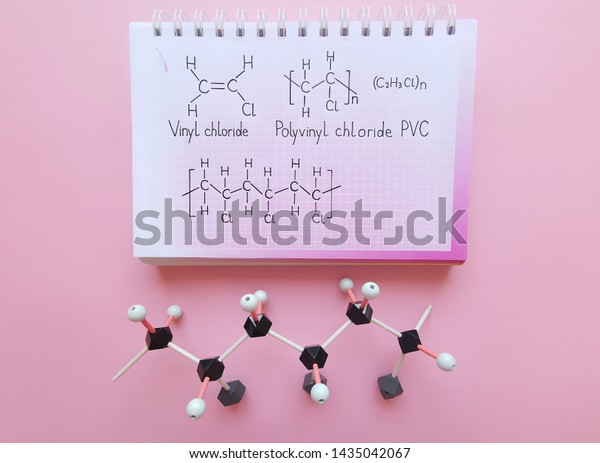
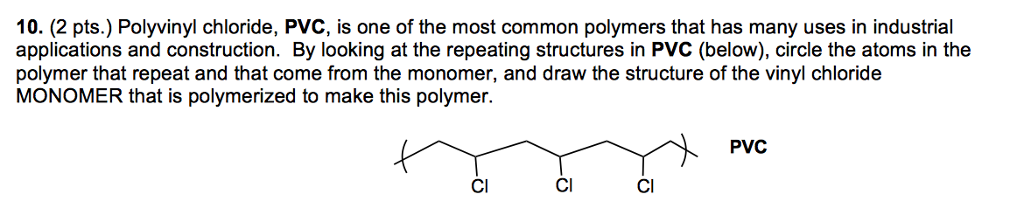
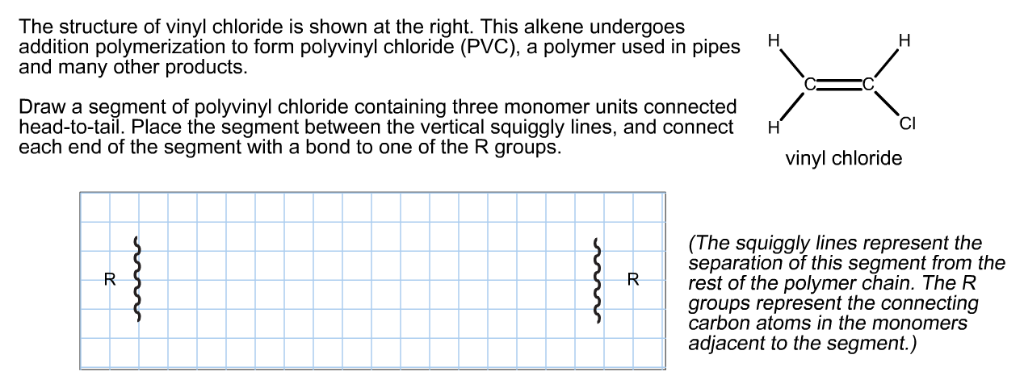
Vinyl chloride monomer structure. Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics. Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene about 40 million tons of pvc are produced each year. Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer. It is a clear colorless liquid with a sweet fruity smell.
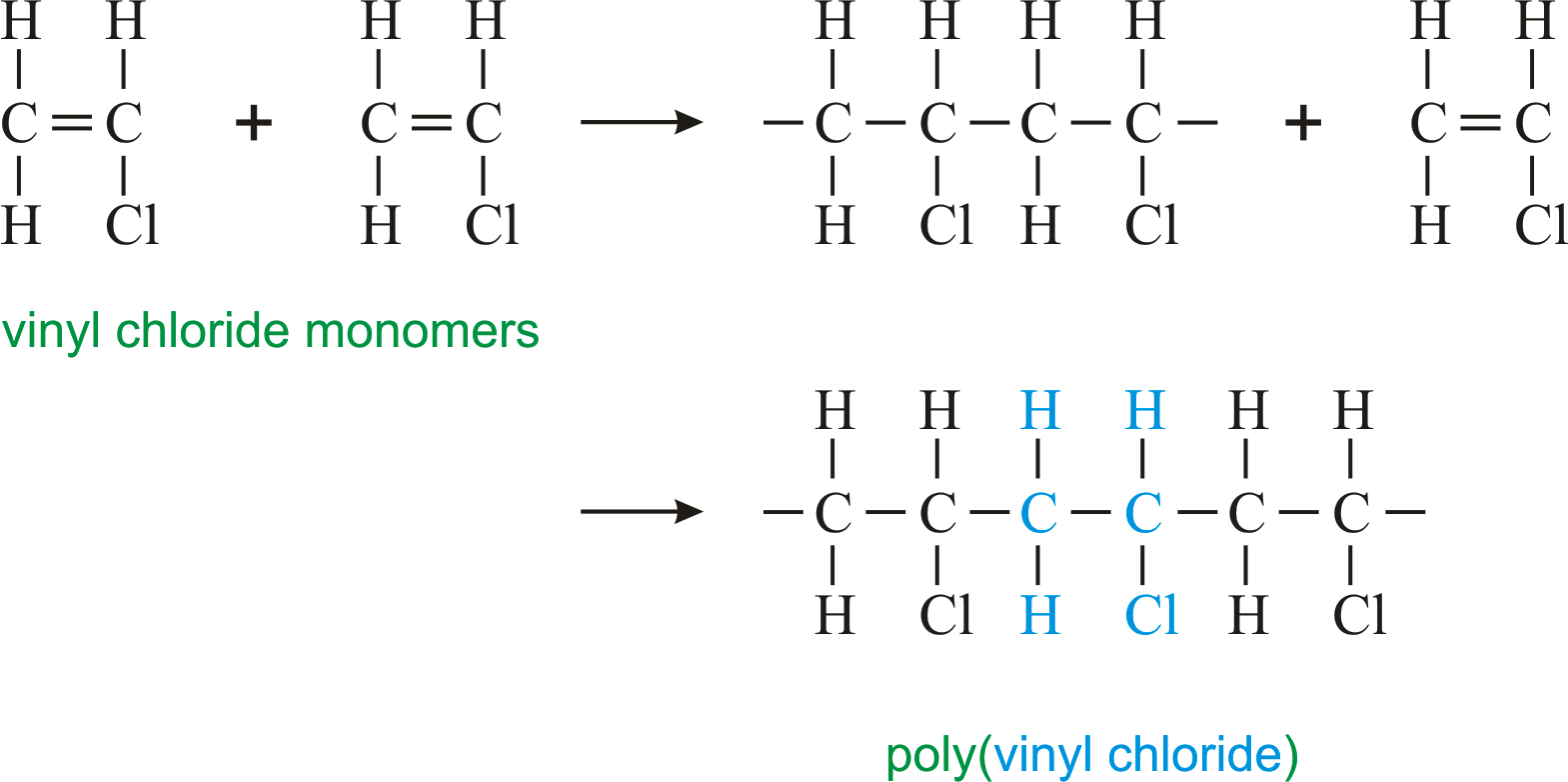
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc. Vinyl chloride monomer vcm is prepared commercially in two processes based on different two carbon hydrocarbons. Polyvinyl chloride is a white rigid quite brittle solid. Vinyl acetate is used to make other industrial chemicals.
Rigid sometimes abbreviated as rpvc and flexible. Synthesis of vinyl chloride. The substance identity section is calculated from substance identification information from all echa databases. Pvc comes in two basic forms.
Vinyl chloride is also produced as a combustion product in tobacco smoke. Substance identity substance identity. About 13 billion kilograms are produced annually. Vinyl acetate is an industrial chemical that is produced in large amounts in the united states.
Vinyl chloride is an organohalogen compound that has important industrial applications. Vinyl chloride is used primarily to make polyvinyl chloride pvc. The balanced process is based on ethylene and the carbide process is based on acetylene. Suspension and emulsion polymerization of vinyl chloride monomer using free radical initiators.
Pvc is used in the manufacture of numerous products including packaging films and water pipes. It is very flammable and may be ignited by heat sparks or flames. Pvc is used to make a variety of plastic products including pipes wire and cable coatings and packaging materials. Vinyl chloride ch 2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst.
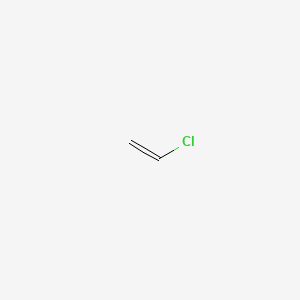
It is a carcinogenic gas that must be handled with special protective procedures. Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.